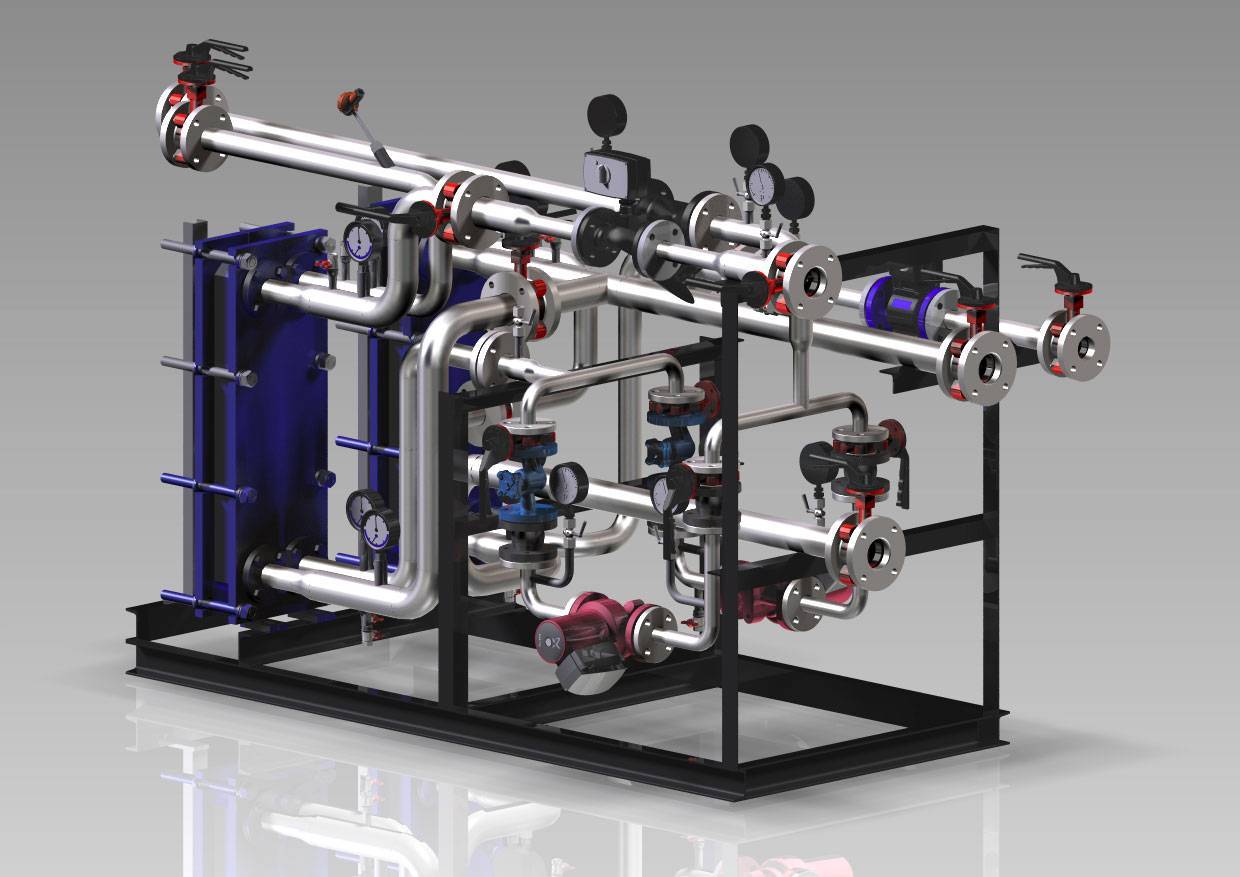
Проектирование и монтаж ИТП в Москве: ключевые аспекты и лучшие практики
Индивидуальные тепловые пункты (ИТП) играют критическую роль в системе отопления, горячего водоснабжения и вентиляции зданий. В Москве, городе с холодными зимами и высокими требованиями к комфорту жилья, вопросы проектирования и установки ИТП стоят особенно остро.